In Japan, plans are under way to promote the use of fuel ammonia to generate electricity from thermal power generation. This article will focus on nitrogen, an essential component in ammonia production, and look at the impacts of the massive use of ammonia on the natural nitrogen cycle.
Natural nitrogen cycle
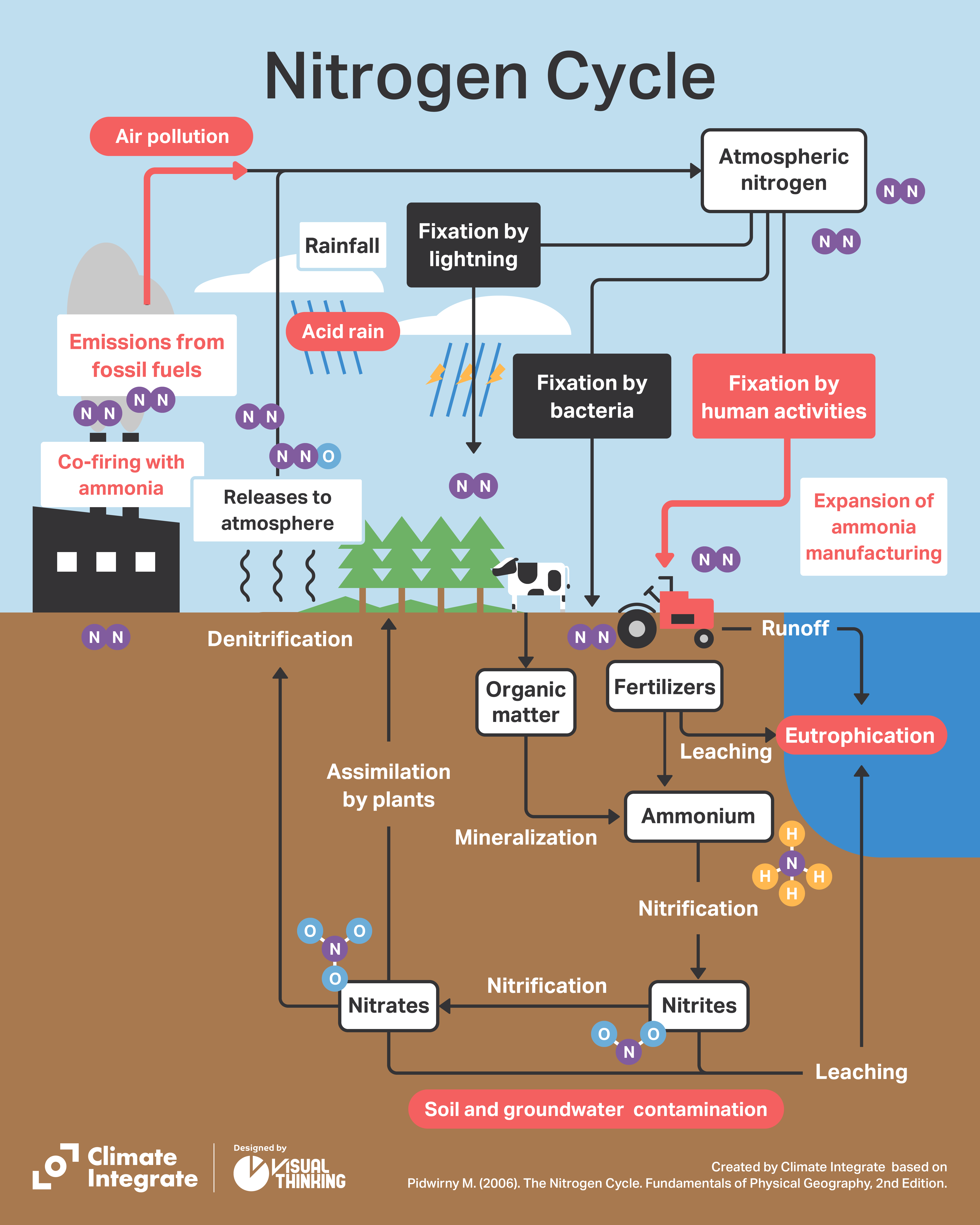
Ammonia (NH3) is a compound consisting of nitrogen and hydrogen. Nitrogen accounts for 78% of the Earth’s atmosphere, and is one of the elements in the proteins that are essential for living organisms. In the atmosphere, it exists in the form of extremely stable nitrogen molecules (N2). Living organisms cannot easily use nitrogen, unlike oxygen, but certain microorganisms in ecosystems have the function of “fixing” nitrogen from the atmosphere and combining it with hydrogen and carbon. Rhyzobia is a well-known group of soil bacteria that has a symbiotic relationship with the roots of leguminous plants. Because legumes fix nitrogen in a form that can be used by living organisms, they have been planted together in fields to help crops grow.
The nitrogen fixed by microorganisms is taken up by plants mostly in the form of ammonium ions and nitrate ions, and converted into amino acids and proteins, etc. Only through ingestion can animals incorporate the nitrogen into their bodies. When plants and animals die, the nitrogen that was in the plant or body breaks down again through the action of microorganisms, is reduced to inorganic nitrogen and released back into the atmosphere as N2. That describes the nitrogen cycle, which is so important in maintaining ecosystems on Earth.
In ecosystems there is a close balance between the amount of atmospheric nitrogen that is fixed by microorganisms, and the amount of nitrogen converted back to gaseous N2 and returned to the atmosphere. This is how the N2 concentration in the atmosphere and the amount of nitrogen on Earth have remained stable for eons.
Advent of the Haber-Bosch process for producing ammonia from nitrogen
The only way to produce ammonia was from minerals such as nitratine (a.k.a. Chile saltpeter) until two German chemists, Fritz Haber and Karl Bosch, were able to artificially fix nitrogen from the atmosphere, enabling its use as nitrogen fertilizer and other applications. In 1909, Haber developed a method to artificially synthesize ammonia from nitrogen in the atmosphere, using special catalysts under high pressure and temperature, at 175 atmospheres and 550 degrees Celsius. The reaction itself was simple: N2+3H2 → 2NH3. But it was very difficult to make this occur efficiently. However, at German chemical company BASF, Bosch succeeded in developing industrial processes based on Haber’s theory through improvements in catalysts, etc., and in 1913 a plant started producing ammonia in large quantities. What he used is known as the Haber-Bosch process, an ammonia production technology that has been in use now for over 100 years.
This technology enabled a dramatic increase in agricultural production. At the time, hydrogen was produced by reforming coal, so this was referred to as “a way to make bread out of air.” For their achievements, Haber received the Nobel Prize in Chemistry in 1918, and Bosch in 1931.
Disrupted nitrogen cycle
Thus, for over a century nitrogen in the atmosphere has been fixed and supplied in the form of fertilizer and industrial ammonia. Added to that, fossil fuels containing not only carbon but also nitrogen have been burned in vast quantities for more than 150 years, releasing nitrogen oxides into the atmosphere. The result has been a massive disruption of the nitrogen cycle.
While the annual amount of nitrogen fixation by living organisms (biological fixation) is approximately 54 million tons, artificial nitrogen fixation is estimated at as much as 30 million tons. If we add combustion and other factors, the total amount of nitrogen released annually into the environment by humans is about 107 million tons, far exceeding the amount of nitrogen fixed.
Environmental impacts
Human interference in the natural nitrogen cycle has triggered many environmental problems.
・Air pollution, acid rain, photochemical smog
Nitrogen oxides (NOx) from the combustion of fossil fuels cause air pollution. NOx is also a cause of acid rain, as it is taken up into cloud droplets and atmospheric particles, and after a series of chemical reactions, falls as highly acidic rain and acid deposition. In addition, photochemical oxidants such as ozone are generated through photochemical reactions between hydrocarbons and the energy of ultraviolet light from the sun. This is a cause of photochemical smog.
NOx emissions in Japan have been declining in recent years and are now at about half of what they were at the peak in 2005. Despite that, there has been almost a complete failure to meet environmental standards. In 2020, only 0.2% of ambient air pollution monitoring stations (and 0% of roadside stations measuring vehicle exhaust) met environmental standards.
・Marine eutrophication
Serious environmental problems are also being caused by large amounts of nitrogen compounds in aquatic environments such as rivers and oceans. When large amounts of nitrogen and phosphorus flow into enclosed coastal seas such as the Seto Inland Sea or Tokyo Bay, phytoplankton feeding on the nutrients multiply rapidly, resulting in eutrophication. A typical example of this can be seen in large algae blooms in Kasumigaura (Japan’s second largest lake) in Ibaraki Prefecture. Excessive growth of algae blooms consumes a large amount of oxygen in the water, resulting in die-offs of fish and other aquatic life. If river or lake water is being used as drinking water, algae blooms can prevent water intake, or result in offensive odors and taste.
In 2011, the World Resources Institute (WRI) and Virginia Institute of Marine Science (VIMS) published a map of 530 dead zones and 228 marine zones that have severe eutrophication. Their total area amounts to 243,000 km2, comparable to the size of New Zealand. The world’s dead zones have reportedly quadrupled in area since 1950.
・Groundwater contamination
Nitrogen entering groundwater also causes other problems. Some of the nitrogen used in farmland becomes nitric acid, which penetrates underground and becomes nitrate nitrogen. Nitrite nitrogen, a type of nitrate nitrogen, oxidizes hemoglobin in blood cells and turns it into methemoglobin. Since methemoglobin is unable to bind to oxygen, oxygen in the blood decreases, causing an oxygen deficiency known as methemoglobinemia. Drinking nitric acid-contaminated groundwater causes disease in infants and others, which is why governments establish water quality standards for tap water.
In the 1960s, nitrate pollution mainly from extensive use of nitrogen fertilizers on farmland was discovered in groundwater in Kakamigahara City, Gifu Prefecture. Contamination was detected at levels far exceeding tap water quality standards. The problem was mitigated somewhat by improvements in methods to apply fertilizer, but a 2017 survey of groundwater quality by the Ministry of the Environment found that nitrate nitrogen and nitrite nitrogen exceeded environmental standards by 2.8%, the highest ever. The number of wells with levels exceeding environmental standards has increased rapidly since 1999, and no improvement in the groundwater pollution has been observed.
Warnings from scientists
The Millennium Ecosystem Assessment (MEA), an international scientific project led by the United Nations from 2001 to 2005, found that the supply to terrestrial ecosystems of nitrogen and phosphate compounds such as fertilizers has doubled since 1960, and pointed out the threat of significant impacts on biodiversity from the excessive buildup of nitrogen.
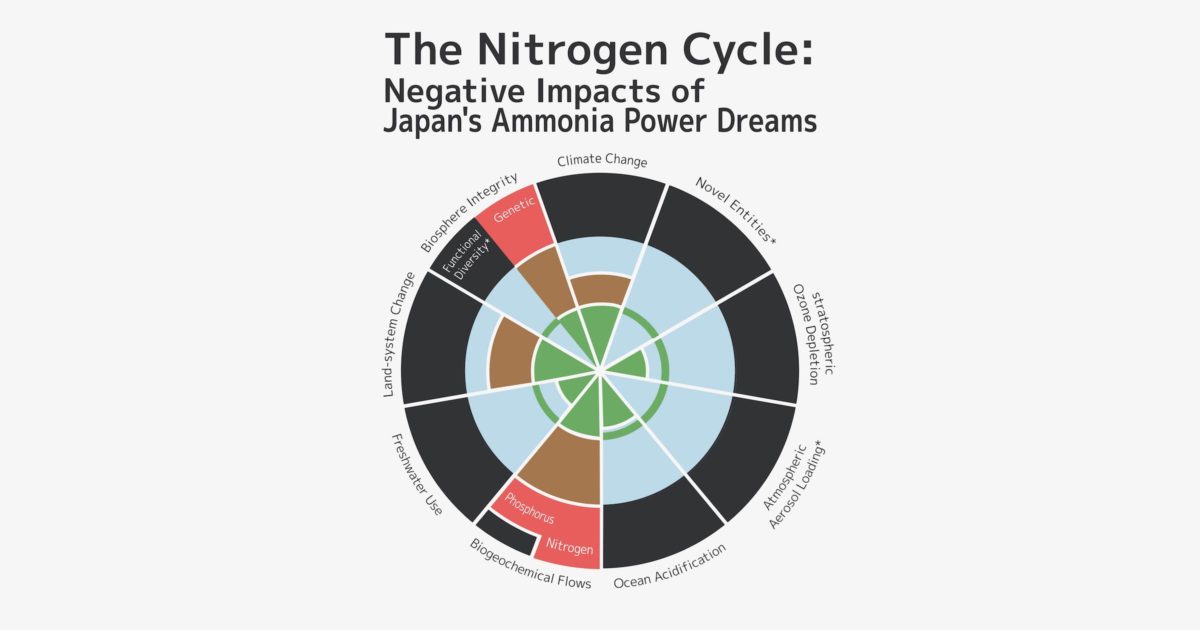
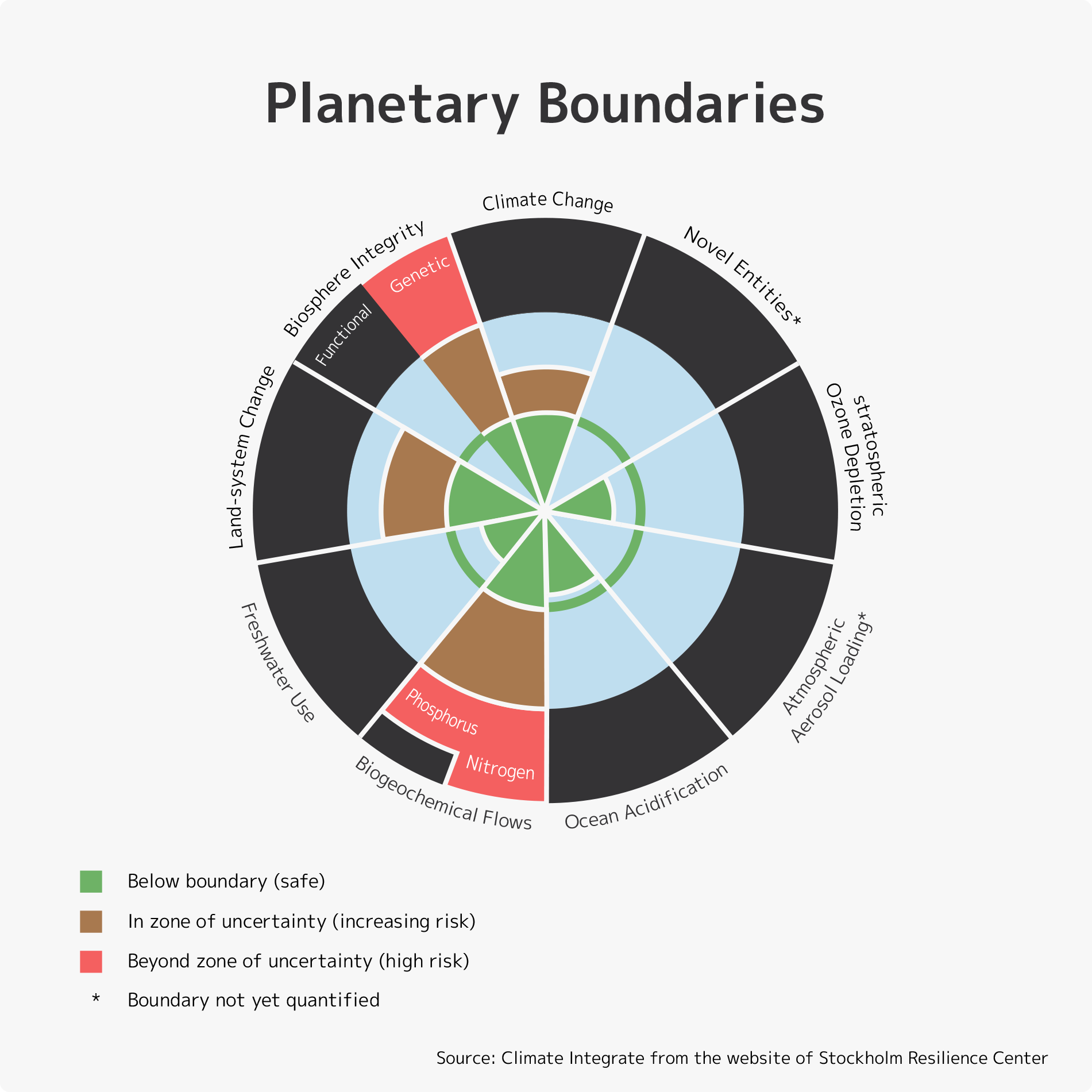
In 2009, a group of scientists led by Johan Rockström, an environmental scientist at the Stockholm Resilience Center, and Will Steffen, a chemist at the Australian National University, in a study on “Planetary Boundaries” found that among nine ecosystem limits, nitrogen pollution exceeded boundaries the most. Their report points out that “Human activities now convert more N2 from the atmosphere into reactive forms than all of the Earth’s terrestrial processes combined. … Although the primary purpose of most of this new reactive N is to enhance food production via fertilization, much reactive N eventually ends up in the environment—polluting waterways and coastal zones, adding to the local and global pollution burden in the atmosphere, and accumulating in the biosphere.”
Use of fuel ammonia for thermal power generation
Now, back to the issue of using ammonia for thermal power generation. Various issues have been pointed out, including the fact that the burning of ammonia to generate electricity does not help reduce CO2 emissions, because it requires hydrogen obtained by reforming fossil fuels. Plus, expanded use of the Haber-Bosch process, which requires high temperature and high pressure, will lead to an increase in energy consumption.
Even with innovations, as long as the Haber-Bosch process is being used to fix atmospheric nitrogen to produce ammonia, the use of ammonia as an alternative fuel for thermal power generation is not unrelated to human disruptions of the nitrogen cycle. In fact, it would interfere further with the already very unbalanced nitrogen cycle, further increasing the impacts on land and water systems.
Furthermore, if ammonia is to be burned in thermal power generation, the magnitude of the NOx emissions problem cannot be ignored. Ammonia is carbon-free at the moment of combustion because it does not contain carbon (C), but it does emit much NOx and can also produce nitrous oxide (N2O), a powerful greenhouse gas.
Low oxygen combustion: NH3+2O2 → HNO3+H2O
High oxygen combustion: 4NH3+5O2 → 4NO+6H20
To prevent air pollution from NOx, powerful denitrification equipment is required. Based on its R&D, the Central Research Institute of Electric Power Industry claims that NOx is not a major problem if the co-firing rate is about 20% ammonia, but problems with higher co-firing ratios have not been solved at all. The Ministry of Economy, Trade and Industry website acknowledges the problem: “Demonstration tests so far have shown that if ammonia is co-fired at 20%, the NOx value in the exhaust can be maintained at the same level as when only coal is burned. However, only small test furnaces have been used to date, so it will be necessary to expand the scale and conduct more demonstration trials using actual facilities in order to achieve practical use.”
As described above, nitrogen fixation and any expansion in the use of ammonia poses a variety of serious problems. Currently, the Japanese government is promoting the use of fuel ammonia, and there are projections that annual global demand for ammonia will quadruple from the current 200 million tons to 760 million tons by 2050. What we actually need to do is rapidly reduce the nitrogen load on ecosystems. It is already beyond the natural carrying capacity, but the greater use of ammonia would greatly exacerbate the nitrogen problem.
In view of ecological principles and the fact that humanity has already significantly disrupted the nitrogen cycle over the past 100 years, we need a comprehensive and long-term perspective of sustainability and global environmental issues, and must reconsider policies that are promoting the use of ammonia.
Written by Tetsuji Ida (Kyodo News)
Design: Jun Sakurada (Visualthinking), Yasuyuki Sasaki